Don't Let Incorrect Denials Sidetrack Your SNS Coding
Stages and modifiers are your keys to success.
Patients sometimes are scheduled for sacral nerve stimulation (SNS) to correct urinary incontinence when other, more conservative treatments have failed (such as bladder retraining, diet modification, or medication). Coding the procedures is straightforward, but can sometimes lead to incorrect denials when the payer overlooks important details. Read on to be sure you’re reporting everything correctly.
Understand the Procedure Itself
Sacral nerve stimulation involves electrical stimulation of the nerves that control the bladder. Nerve stimulation can address symptoms of overactive bladder including:
During the procedure, the surgeon implants a neurotransmitter device under the skin in the upper buttock area. The device transmits mild electrical impulses through a lead wire close to the sacral nerve in the lower back; these impulses, in turn, influence the bladder sphincter and pelvic floor muscles to provide bladder control.
Important to remember: Sacral nerve stimulation will not cure these bladder control problems, but it can reduce the number of voids and/or the number of wetting episodes. The treatment can be completely reversed and discontinued at any time without permanent damage to the patient’s nerves or spinal cord.
Prepare to Code Multiple Encounters
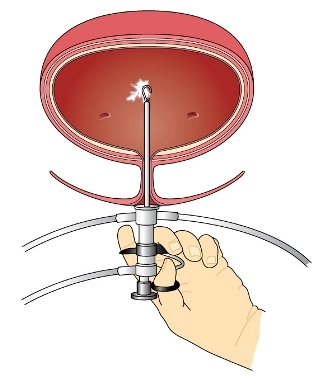
Before the actual device is implanted, a temporary test stimulation lead is placed –this party of the procedure is known as Stage I. This simple outpatient operation begins a one- to two-week trial of the device (also using a temporary external generator) to show how SNS affects the patient’s bladder control symptoms during everyday activities.
On some occasions during the testing phase because a temporary lead may become dislodged and fall out, the urologist may implant one lead (under fluoroscopic guidance) as a permanent lead. The lead again is connected to a temporary external generator so your physician can test it before implanting the permanent generator.
Common scenario: For Stage I testing the urologist most often places a temporary lead and therefore bills code 64561 (Percutaneous implantation of neurostimulator electrode array; sacral nerve [transforaminal placement] including imagine guidance, if performed) for the temporary lead. As indicated by the descriptor, fluoroscopic guidance is included and not separately billable with 64561.
Another option: If the urologist places a permanent lead initially, then you should report code 64581 (Incision for implantation of neurostimulator electrode array; sacral nerve [transforaminal placement]) for the permanent lead placement. Also include 77002-26 (Fluoroscopic guidance for needle placement [eg, biopsy, aspiration, injection, localization device]; Professional component) for the fluoroscopic guidance used for the permanent lead implantation. The procedure usually is performed in the hospital setting.
If the trial placement is a success (meaning a 50 percent reduction in patient symptoms), the patient will return for Stage II of the procedure – placement of the long-term devices. Report this placement with 64581 if a previous temporary lead placement was used. Several days later a permanent generator is implanted into a pouch constructed in the buttocks. Bill this part of the procedure with 64590 (Insertion or replacement of peripheral or gastric neurostimulator pulse generator or receiver, direct or inductive coupling).
Modifier reminder: Unlike the temporary lead implantation code, which has a 10-day global period, the permanent lead implantation code carries a 90-day global period. That means you should attach modifier 58 (Staged or related procedure or service by the same physician or other qualified health care professional during the postoperative period) to 64590 if you are within the 90-day global period, which experts say often happens.
Plus: When reporting the permanent placement, you also need to include a code for programming the generator. Use 95971 (Electronic analysis of implanted neurostimulator pulse generator system [e.g., rate, pulse amplitude, pulse duration, configuration of wave form, battery status, electrode selectability, output modulation, cycling, impedance and patient compliance measurements]; simple spinal cord, or peripheral [i.e., peripheral nerve, sacral nerve, neuromuscular] neurostimulator pulse generator/transmitter, with intraoperative or subsequent programming) or 95972 (… complex spinal cord, or peripheral [i.e., peripheral nerve, sacral nerve, neuromuscular] [except cranial nerve] neurostimulator pulse generator/transmitter, with intraoperative or subsequent programming).
Beware of Incorrect Denials
During the past couple of years, some urology practices noticed incorrect denials for SNS to treat urinary incontinence. Digging into the situation revealed that some payers were incorrectly denying the claims due to code confusion.
“We’ve had trouble with Noridian denials, and them mapping to incorrect LCDs (local coverage determinations),” says Christy Shanley, CPC, CUC, finance director and billing manager for UC Irvine Health’s department of urology. “Their LCD for programming (code 95972) was tied to a diagnosis of Parkinson’s disease. We recently spoke with a manager from Medtronic who is working with Noridian to resolve the issue.”
Here’s why: The same CPT® codes also are included in coding edits in effect for deep brain stimulation to treat essential tremor and Parkinson’s disease and in vagus nerve stimulation. The affected CPT® codes included:
CMS issued a Medlearn Matters article in October 2015 to address updates for NCD editing that contain ICD-10 diagnosis/procedure codes, plus all associated coding infrastructure. If you notice any of these incorrect denials crossing your desk, contact your local CMS representative.