Use this three-step process for properly coding P0100.
Are you certain that your staff use the correct process for using restraints on residents? Keep in mind that Item P0100 — Physical Restraints topped the list of problem areas during the 2014 pilot MDS-focused surveys. With the Centers for Medicare & Medicaid Services (CMS) gearing up to launch nationwide surveys in 2015, you need to keep your coding and compliance airtight.
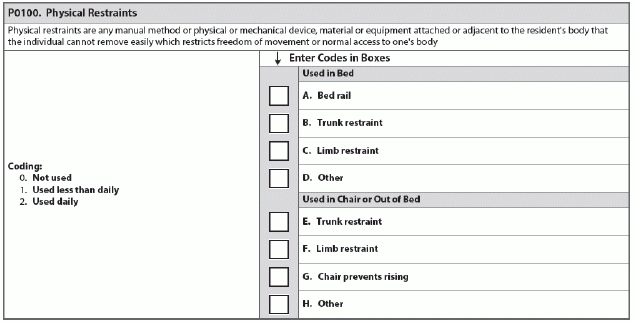
The RAI Manual defines “physical restraints” as: “Any manual method or physical or mechanical device, material or equipment attached or adjacent to the resident’s body that the individual cannot remove easily, which restricts freedom of movement or normal access to one’s body.”
1. Don’t Use the Wrong Process
When you’re coding Item P0100, ensure that you’re using the correct process for determining whether staff are using “restraints” on a resident. According to the University of Missouri-Columbia (UM-C) Sinclair School of Nursing, you should use the following three-step process:
1. Evaluate whether the resident can easily remove the device, material or equipment.
2. If not, does the device meet the other provisions in the definition of restricting freedom of movement? What effect does the device have on the resident?
3. Consider other devices or situations that may have the effect of a restraint but do not fit into the current P0100 categories, such as a low bed that causes a resident to now need assistance to get up when he didn’t before. You would code these as “Other” in P0100.
2. Don’t Think About Intent
Next, determine whether the device is listed in P0100. If the device is listed, determine the effect it has on the resident, not the device’s intent.
You would code in P0100 if the device prevents the resident from attempting or completing an activity that he could do if the device wasn’t present, or if the device limits the resident’s access to his body, UM-C instructs.
But you should not code in P0100 if the device doesn’t restrict the resident from attempting or completing an activity that he could do if the device were not present.
You must code only the devices that have the effect of restraining the resident, said Ann Spenard, MSNSN, RN, C, vice president of consulting services for Wethersfield, Conn.-based Qualidigm, in a CMS instructional session. You must focus on the effect on the resident, not the device’s intent.
For instance, an “enabler” may still have a restraining effect, which you must then code as a restraint, UM-C states.
3. Don’t Blunder Your Documentation
If the device or situation meets the definition of a restraint, you must have a physician’s order and medical reason for the restraint in the documentation, UM-C stresses. You should also record the team evaluation and process for how staff decided to use that particular device for that resident instead of another device or situation.
Also, document the conversation when informing the resident’s family of the device’s risks and benefits.
4. Don’t Forget About the Care Plan
Yet another crucial part is the care plan for the resident when you’re using restraints. Care plan for the effects of any device or situation, even if it doesn’t meet the definition of a restraint, UM-C instructs. Make sure the care plan reflects specific care issues.