OASIS height and weight data collection poses a challenge.
The Centers for Medicare & Medicaid Services has plans to move forward with a new quality measure that focuses on new or worsened pressure ulcers.
Quality measure NQF #0678, Percent of Residents or Patients with Pressure Ulcers that are New or Worsened (Short Stay) in the [Home Health Quality Reporting Program] will function as a cross-setting quality measure to meet the requirements of the IMPACT Act for the CY 2018 payment determination and subsequent years, CMS said in the home health prospective payment system final rule published in the Nov. 5 Federal Register.
“This measure reports the percent of patients with Stage 2 through 4 pressure ulcers that are new or worsened since the beginning of the episode of care,” according to the rule.
Multiple commenters on the proposed rule expressed support for focusing on the area of pressure ulcers. Implementing the measure will “help facilitate best practice measures for prevention of pressure ulcers and appropriate care for pressure ulcers in all settings,” said Barbara Dale of Tennessee in her comment letter. “Pressure ulcers in the home ARE devastating to patients and caregivers alike.”
Bonus: CMS will draw the data for this measure from items already collected in OASIS: M1308 — Current Number of Unhealed Pressure Ulcers at Each Stage or Unstageable and M1309 — Worsening in Pressure Ulcer Status Since SOC/ ROC, according to the final rule. Data for risk adjustment also is already collected via OASIS, CMS notes in the rule. OASIS items used would most likely include M1850 — Activities of Daily Living Assistance, Transferring, M1620 — Bowel Incontinence Frequency, M1016 — Diagnoses Requiring Medical or Treatment Change Within past 14 Days, M1021 — Primary Diagnoses, and M1023 — Other Diagnoses. CMS began collecting the M1309 data last January, in anticipation of coming IMPACT Act measures.
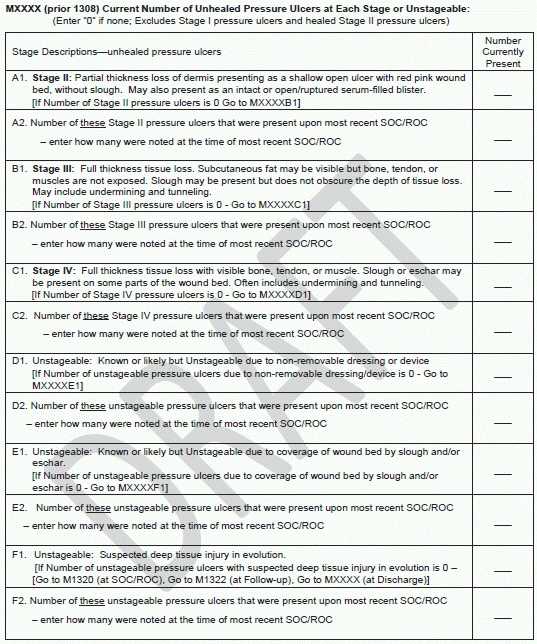
Sneak peek: But there would be some changes to the integumentary status items that would support this measure, and CMS would renumber M1308 and M1309 in implementing the revisions. Proposed changes to M1309 include rewording the instructions to read “Indicate the number of current pressure ulcers that were not present or were at a lesser stage at the most recent SOC/ROC. If no current pressure ulcer at a given stage, enter 0.”
CMS plans to change M1308 to collect data about the number of pressure ulcers at each stage that were present at the most recent start of care or resumption of care.
See a draft of all the changes at www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HomeHealthQualityInits/Downloads/OASIS-January_2017_July2015.pdf .
CMS plans to add an M item that will ask whether patients had certain diagnoses that might put a patient at risk of developing pressure ulcers in the last seven days. The draft item instructs agencies to check one or both boxes that apply: “1 — Peripheral Vascular Disease (PVD) or Peripheral Arterial Disease (PAD)” and/or “2 — Diabetes Mellitus (DM) (e.g., diabetic retinopathy, nephropathy, and neuropath.” The item includes the instruction that “Diagnoses listed in parentheses are provided as examples and should not be considered as all-inclusive lists.”
CMS also plans to add another M item to collect a patient’s height and weight, in order to calculate body mass index (BMI) as a risk adjustor for this new quality measure.
Note: For more tips and strategies on running a successful home health agency, see Eli’s Home Care Week. Information on subscribing is online at www.codinginstitute.com/newsletters/eli-home-care-week.html .
